
|
|
|
 | |
 |
 |
|
| ||||
|
| |||||||
|
|
Research is the foundation upon which the Meinwald Group is built. As past, ongoing, and future projects are adapted to web format, they will join the ranks of the research represented on these pages.
Chemical Defense of Ladybug Pupae:A Combinatorial Library of Macrocylic AminesLadybugs are cute and harmless, right? Even beneficial? Well, not Epilachna varivestis, the Mexican bean beetle, and Epilachna borealis, the squash beetle, orange-yellow ladybugs with a few dark spots on their backs. 
An adult Epilachna borealis feeding on a squash leaf, next to it a brightly yellow-colored larva. The pupae of these two species (both are among the few ladybug species that are pests to farmers) have puzzled entomologists in that they appear to have no mechanical defenses, are bright yellow in color and therefore highly conspicious, and yet manage to thrive. Many ladybug larvae have mechanical defenses including fake mandible-like extensions on their abdomens, which they wiggle and pinch their enemies with. Epilachna pupae use other means. Their surface is covered with a dense coating of glandular hairs, each bearing an oily droplet at its tip ((PIC)). This secretion is potently deterrent against ants, one of the pupae’s most persistent enemies. Ants who come in contact with it recoil instantly and try frantically to wipe the secretion off their forelegs and antennae. 
(A-C) Pupa of C. sanguinea responding to stimulation with bristle of a fine paint brush. The jaw-like "gin traps" on the back of the pupa are ordinarily held agape (arrows in A). Insertion of the bristle into a trap causes the pupa to flip upward, with the result that the bristle is "bitten". (D) Pupa of Mexican bean beetle (E. varivestis), in dorsal view. Note the glandular hairs, with glistening droplets of secretion at the tip, that fringe the pupa. (E) Enlarged view of glandular hairs of E. varivestis pupa. (F) Ant (Crematogaster cerasi) that has just contacted the glandular hairs of an E. borealis pupa (left) with an antenna, cleaning that antenna by brushing it with a foreleg. In E. varivestis (the Mexican bean beetle) the active principles of this secretion are azamacrolides, novel lactones with a single nitrogen atom incorporated into a ring of 14, 15, or 16 members. These compounds can easily be detected using gas chromatographic techniques. The major component is epilachnene 1. 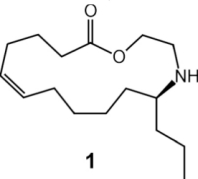 Surprisingly, gas chromatographic analyses of the pupal secretion of the squash beetle, E. borealis, indicated only the presence of vitamin E acetate (2) and other tocopherol derivatives. The tocopherols did not appear as likely candidates for insect repellents, and in fact, tests with ants showed that these compounds proved to be essentially inactive, while the secretion itself was potently deterrent. To find and identify the active components in the E. borealis secretion, we therefore adopted a more general analytical approach. Direct NMR-spectroscopic analyses revealed an unprecedented – and entirely unexpected – degree of complexity. The tocopheryl acetates account for only a relatively small percentage of the beetles' total secretion (~20%), while the major components represented a group of previously undetected compounds. These components were shown to be esters and amides derived from the carboxyl- and the 2-hydroxyethylamino moieties of the three (2-hydroxyethylamino)alkanoic acids, 3-5, which occur in a ratio of 2.5:8.5:89. 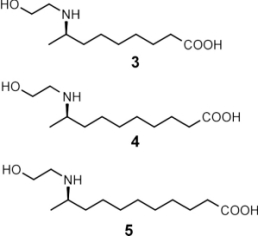
The three building blocks 3-5 of the macrocyclic polyamines 6-11 From the complexity of the 1H-NMR spectra of the major components in the secretion, it was apparent that the three homologous (w-1)-(2-hydroxyethylamino)alkanoic acids 6-8 must represent the "building blocks" of large number of structurally similar cyclic esters and amides. Thus, the new E. borealis secretion components appeared to consist of higher molecular weight oligomers, each combining several (w-1)-(2-hydroxyethylamino)alkanoic acid units. This hypothesis was corroborated by HPLC-MS analyses using positive-ion electrospray ionization, which revealed the secretion to contain a highly convoluted mixture of macrocyclic polyamines.
Macrocyclic amines: the trimers 7, tetramers 8, and pentamers 9 represent the major components, accompanied by smaller amounts of dimers 6, hexamers 10, and heptamers 11. In these formulas, each of the variables m ... s can have the values 5, 6, or 7. In summary, the Epilachna borealis pupal secretion consists largely of a combinatorial library of macrocyclic polyamines with very large ring sizes of up to 200 members, corresponding to the set of bis- to hepta-lactones 6-11 accompanied by their various lactam isomers and smaller amounts of higher oligomers. Judging from the quantitative distribution of homologues in each oligomeric series, it appears that the three building blocks, 3-5, are incorporated into these oligomers in random fashion. To our knowledge, the range of ring sizes displayed by the PAML's is unparalleled by any other group of secondary metabolites, and is reminiscent of the large cyclic oligopeptides such as the conotoxins or cyclic oligosaccharides such as the cyclodextrins.
|